Probiotics: Strong Foundations Stemming from Birth
There is a lot of talk about probiotics these days, and for good reason. These incredible bacteria have been shown to fight off infections, decrease inflammation, improve mood, clear up skin, and help cardiovascular issues. You name it, they can do it! I would like to bring your attention to two unique and very important foundational bacteria: B. infantis and L. reuteri.
 B. infantis
B. infantis
Starting at the beginning of life, Bifidobacterium longum ssp. infantis—yes, its full name is quite a mouthful—is abundant in breastfed babies. At birth, we get a huge inoculation of bacteria as we pass through the vaginal canal; then, breast-feeding continues to provide a unique microbiota consisting of millions of bacteria per day.[1] Breast milk is a “magical superjuice” that is impossible to recreate. Not only is it packed with all the nutrients a baby needs, but it also contains beneficial bacteria as well as carbohydrate fibres called oligosaccharides. Humans are unable to digest these oligosaccharides,[2] but B. infantis can—it is the only probiotic able to digest the complete array of oligosaccharides in breast milk. Therefore, it has a major advantage over other bacteria.[3] This helps it grow in abundance, making up about 80–90% of a baby’s microbiota.[4], [5] Bifidobacteria in general—and especially B. infantis—ferment milk oligosaccharides, producing short-chain fatty acids (SCFAs) such as acetate and butyrate.
SCFAs nourish the cells of the intestines, provide nutrients for the growing baby, and act to fight off pathogens. B. infantis is a foundational bacterium that colonizes the intestine and supports the growth of other friendly bacteria.
Preterm infants fed B. infantis showed increases in B. infantis, as predicted, but also an abundance of total Bifidobacteria, showing how this probiotic paves the way for others to colonize as well.[6] Bifidobacteria seem to cooperate to breakdown complex carbohydrates into simple sugars, working together as a community[7] and to make more nutrients—in this case, simple sugars—available for other gut bacteria.
Another important role of B. infantis is strengthening the intestinal wall. Babies have spaces between their intestinal cells, and therefore more permeability for pathogens to enter the lymph and bloodstream. B. infantis signals the intestinal cells to produce proteins to fill these gaps, reducing the permeability and strengthening the intestinal wall.[8] Folate (vitamin B9) can also be produced by B. infantis, which is vital in the production of red blood cells and oxygen transport to tissues, and has a major role in DNA synthesis and repair.[9]
B. infantis is valuable at all ages as it has an incredible ability to repair the intestinal wall and reduce inflammation, being helpful in conditions such as ulcerative colitis.[10], [11] It also plays a major role in the immune system maturation and regulation; therefore, taking B. infantis after antibiotic use or following a colonoscopy can support the body for recovering. Also, as B. infantis has evolved to colonize the infant gut, it has been shown as a safe approach to restoring the gut microbiome of infants born by C-section.[12]
 L. reuteri
L. reuteri
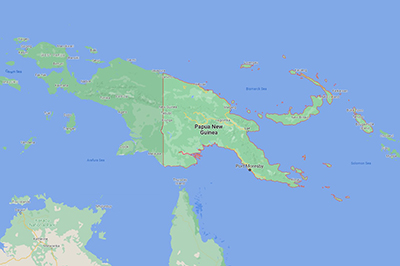
Lactobacillus reuteri is one of our most studied bacteria. It was named after a German microbiologist, Gerhard Reuter,[13] and was first isolated in 1962.[14] In early research, it was regularly found in human digestive tracts and was found in the same subjects on multiple occasions, scientists concluding it is a stable member of the human microbiota.[15] Curiously, current research shows that L. reuteri seems to be absent from our digestive tracts. Comparing subjects from rural Papua New Guinea with those in the United States, L. reuteri was found in the digestive tracts of all subjects in rural Papua New Guinea, but none in the US group.[16]
This obvious decrease in the abundance of L. reuteri in the past few decades is “correlated with an increase in the incidence of inflammatory disease over the same period of time.”[17] It has been hypothesized that this “disappearance” of L. reuteri could be related to dietary changes, medication use, and sanitation practices.[18]
 Overall, L. reuteri is a tough little bug. Like other lactic acid–forming bacteria, it tends to colonize the upper gastrointestinal tract, especially the small intestine, and it appears to be more resilient than other strains since it can survive in quite acidic environments. It produces antimicrobial molecules, which inhibit the colonization of pathogenic microbes (bad bacteria, viruses, fungus, and parasites), and can stimulate the remodeling of a healthy microbiota in your intestines. L. reuteri also has a great ability to benefit our immune system, especially by reducing the production of inflammatory cytokines and by promoting the secretion of regulatory T cells.[19] This balance is beneficial in allergic conditions where the immune system tends to overreact.
Overall, L. reuteri is a tough little bug. Like other lactic acid–forming bacteria, it tends to colonize the upper gastrointestinal tract, especially the small intestine, and it appears to be more resilient than other strains since it can survive in quite acidic environments. It produces antimicrobial molecules, which inhibit the colonization of pathogenic microbes (bad bacteria, viruses, fungus, and parasites), and can stimulate the remodeling of a healthy microbiota in your intestines. L. reuteri also has a great ability to benefit our immune system, especially by reducing the production of inflammatory cytokines and by promoting the secretion of regulatory T cells.[19] This balance is beneficial in allergic conditions where the immune system tends to overreact.
Just like B. infantis, L. reuteri is excellent at protecting the mucosal barrier function. It can decrease intestinal permeability by increasing tight-junction proteins that strengthen the spaces between intestinal cells.[20] Many inflammatory conditions are related to damage within the intestinal wall and possibly the movement of bacteria across it coming into contact with the immune system. L. reuteri may prevent this movement of bacteria across the membrane, improving resistance to inflammatory diseases in the digestive tract as well as in remote tissues.[21]
Conclusion
To summarize, both B. infantis and L. reuteri could prevent infections and strengthen healthy immune responses, therefore being beneficial for allergies or asthma for example. These two strains should also be considered in inflammatory diseases such as ulcerative colitis and other bowel conditions such as irritable bowel syndrome (IBS).[22] Anyone with gut-barrier issues, such as hyperpermeability, could also benefit. For instance, B. infantis and L. reuteri will help with atopic skin conditions such as eczema and psoriasis at all ages. L. reuteri can help in infant colic and with H. pylori, E. coli, and other infections.[23] Breast-feeding moms may consider adding B. infantis to their daily diet. or it could be given directly to babies (spread onto nipple when breast-feeding or mixing into formula), even preterm infants.[24]
 B. infantis and L. reuteri both need to be stored in cold temperatures, so be sure to buy them at retailers that keep them in the fridge, and ensure they remain refrigerated at home. Look for enteric-coated capsules so your stomach acid doesn’t kill them, allowing them to arrive safely in your intestines. This way, you ensure they can do all the wonderful things they are capable of.
B. infantis and L. reuteri both need to be stored in cold temperatures, so be sure to buy them at retailers that keep them in the fridge, and ensure they remain refrigerated at home. Look for enteric-coated capsules so your stomach acid doesn’t kill them, allowing them to arrive safely in your intestines. This way, you ensure they can do all the wonderful things they are capable of.
 Dr. Krista Mackay, BSc, ND
Dr. Krista Mackay, BSc, ND
Krista practices both in Montreal, Quebec, and Montevideo, Uruguay. A busy mom of two boys, she focuses on naturopathic general/family medicine, helping to find a reasonable balance to optimal wellbeing and stress management, including nutrition, herbal medicine, and mind-body work.
kristamackay.ca
[1] Lyons, K.E., C.A. Ryan, E.M. Dempsey, R.P. Ross, and C. Stanton. “Breast milk, a source of beneficial microbes and associated benefits for infant health.” Nutrients, Vol. 12, No. 4 (2020): 1039.
[2] Lyons et al. “Breast milk, a source of beneficial microbes.”
[3] Nguyen, M., H. Holdbrooks, P. Mishra, M.A. Abrantes, S. Eskew, M. Garma, C.-G. Oca, et al. “Impact of probiotic B. infantis EVC001 feeding in premature infants on the gut microbiome, nosocomially acquired antibiotic resistance, and enteric inflammation.” Frontiers in Pediatrics, Vol. 9 (2021): 618009.
[4] Nguyen et al, “Impact of probiotic B. infantis EVC001.”
[5] Wilkinson, T.S. “Immunity to bacteria infections.” Chapter 8 of Section 2 (p. 408–428) in: Rezaei, N., ed. Encyclopedia of Infection and Immunity. Amsterdam: Elsevier, 2022, 3428 p., ISBN 978-0-12-818731-9.
[6] Nguyen et al, “Impact of probiotic B. infantis EVC001.”
[7] O’Callaghan, A., and D. van Sinderen. “Bifidobacteria and their role as members of the human gut microbiota.” Frontiers in Microbiology, Vol. 7 (2016): 925.
[8] Ewaschuk, J.A., H. Diaz, L. Meddings, B. Diederichs, A. Dmytrash, J. Backer, M. Looijer-van Langen, and K.L. Madsen. “Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function.” American Journal of Physiology: Gastrointestinal and Liver Physiology, Vol. 295, No. 5 (2008): G1025–G1034.
[9] Rossi, M., A. Amaretti, and S. Raimondi. “Folate production by probiotic bacteria.” Nutrients, Vol. 3, No. 1 (2011): 118–134.
[10] Zuo, L., K.T. Yuan, L. Yu, Q.-H. Meng, P.C.-K. Chung, and D.-H. Yang. “Bifidobacterium infantis attenuates colitis by regulating T cell subset responses.” World Journal of Gastroenterology, Vol. 20, No. 48 (2014): 18316-18329.
[11] Han, T., X. Hu, K. Li, D. Zhang, Y. Zhang, and J. Li. “Bifidobactarium infantis maintains genome stability in ulcerative colitis via regulating anaphase-promoting complex subunit 7.” Frontiers in Microbiology, Vol. 12 (2021): 761113.
[12] Duar, R.M., D. Kyle, and R.M. Tribe. “Reintroducing B. infantis to the cesarean-born neonate: An ecologically sound alternative to ‘vaginal seeding.’” FEMS Microbiol Letters, Vol. 367, No. 6 (2020): fnaa032.
[13] Britton, R.A. “Lactobacillus reuteri.” Chapter 8 (p. 89–97) in Floch, M.H., Y. Ringel, and W.A. Walker, eds. The Microbiota in Gastrointestinal Pathophysiology. London: Academic Press, 2017, 419 p., ISBN 978-0-12-804024-9.
[14] Mu, Q., V.J. Tavella, and X.M. Luo. “Role of Lactobacillus reuteri in human health and diseases.” Frontiers in Microbiology, Vol. 9 (2018): 757.
[15] Walter, J., R.A. Britton, and S. Roos. “Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm.” Proceedings of the National Academy of Sciences of the United States of America, Vol. 108, Suppl. 1 (2011): 4645–4652.
[16] [No authors listed.] “Rewilding the human gut: Reintroduction of the species Lactobacillus reuteri.” ClinicalTrials.gov. Updated 2020-05-05. Identifier: NCT03501082.
[17] Mu et al, “Role of Lactobacillus reuteri in human health and diseases.”
[18] “Rewilding the human gut.” op. cit.
[19] Mu et al, “Role of Lactobacillus reuteri in human health and diseases.”
[20] Mu et al, “Role of Lactobacillus reuteri in human health and diseases.”
[21] Mu et al, “Role of Lactobacillus reuteri in human health and diseases.”
[22] Zuo et al, “Bifidobacterium infantis attenuates colitis.”
[23] Walter et al, “Host-microbial symbiosis.”
[24] Nguyen et al, “Impact of probiotic B. infantis EVC001.”

 Stores
Stores
